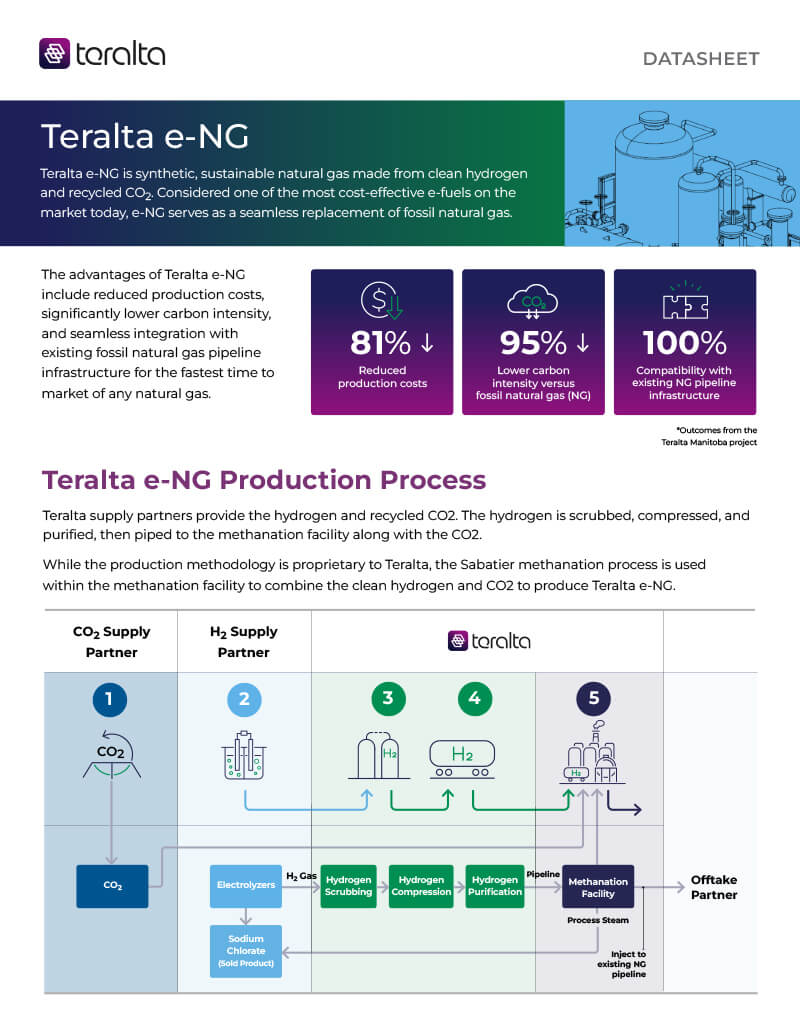
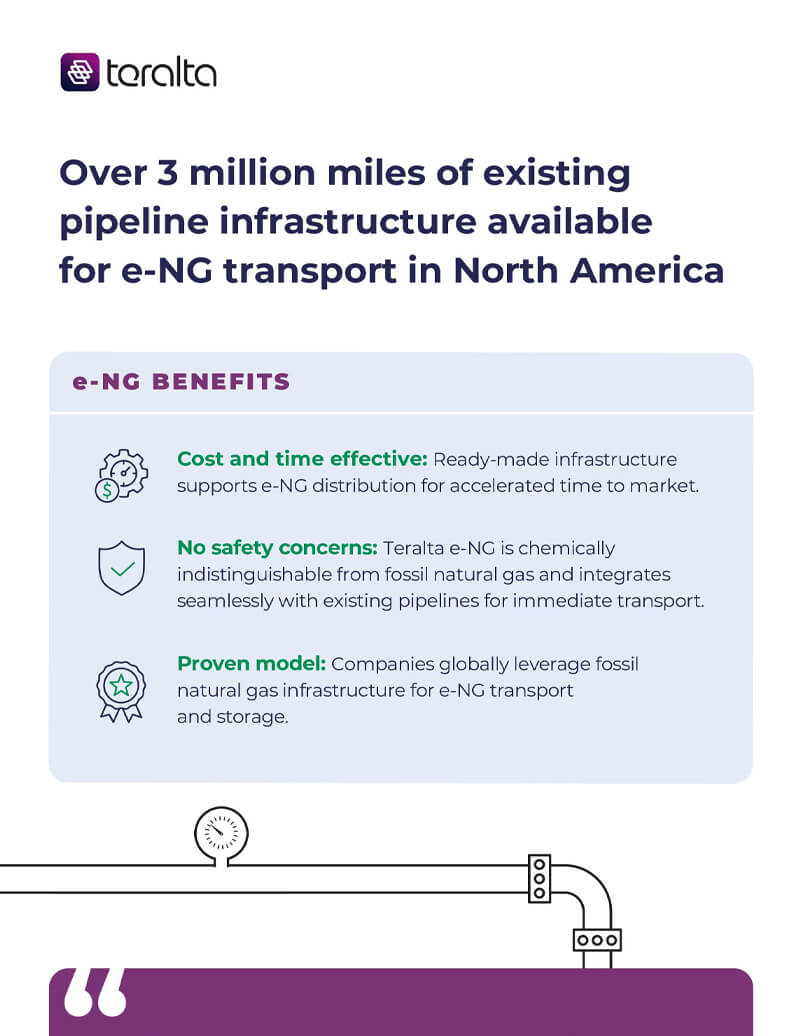
Resources
Access blog posts, press releases, case studies, videos, FAQs and other information about our customers, our people, and our company.
What's New
Get the latest information on Teralta and our hydrogen projects.
Press Release
Tokyo Gas and Teralta Partner to Advance e-Methane Project Development in Canada
Tokyo Gas and Teralta Partner to Advance e-Methane Project Development in Canada Teralta is advancing multiple e-NG project opportunities across North America, including an e-NG project in Brandon, Manitoba, Canada which utilizes by-product green hydrogen ...
December 4, 2025
Press Release
Teralta Joins the e-NG Coalition to Work with Industry Leaders in Achieving a Net-Zero Carbon Future
Teralta Joins the e-NG Coalition to Work with Industry Leaders in Achieving a Net-Zero Carbon Future Teralta Hydrogen Solutions Inc. (“Teralta”), a leader in clean hydrogen and e-NG production within North America, is pleased to ...
January 28, 2025
Press Release
Teralta acquires Loop Energy to advance its clean hydrogen strategy within North America
Teralta acquires Loop Energy to advance its clean hydrogen strategy within North America The acquisition enables Teralta to relocate its head office within the heart of Vancouver’s established hydrogen hub. Facebook X LinkedIn Reddit WhatsApp ...
December 4, 2024
How can we help with your hydrogen project?
Teralta has clean hydrogen and e-NG initiatives actively underway in North America. Contact us if you have an opportunity you’d like to discuss.